バイオメディシン分野において、CAR-T療法はin vitroでの調製からin vivoでのプログラミングへと革命的な飛躍を遂げています。2025年はin vivo CAR-T開発における重要な転換点となります。6月、Capstan社はtLNPベクターをベースとしたin vivo CAR-T療法「CPTX2309」が正式に第I相臨床試験に入ったことを発表しました。その後、AbbVie社はCapstan社を21億ドルの現金で買収し、同社の独自のtLNPプラットフォーム技術を獲得しました。この買収は、大手製薬会社によるin vivo CAR-T療法への高い評価を証明するものであり、この技術が概念実証段階から臨床開発の新たな段階へと正式に移行したことを示しています。
生体内CAR-TとtLNP:精密医療の次のトレンド
業界背景: in vitroからin vivoへの革命的な飛躍
従来の体外CAR-T療法は、B細胞性白血病およびリンパ腫において目覚ましい成果を上げてきましたが、複雑な製造プロセス、高コスト、そして毒性が、この技術の大規模普及のボトルネックとなっています。業界調査によると、市販のCAR-T製品の価格は37万~47万ドルにも達し、細胞採取から再輸注まで3~5週間かかります。病状の急速な進行により、治療を待つことができない患者もいます。
革新的な技術的アプローチを採用したin vivo CAR-T療法は、特殊なベクターを介してCAR遺伝子を体内に直接送達することで、煩雑なin vitro段階を経ることなく、患者の体内で直接T細胞の「機器アップグレード」を完了します。この「体内工場」モデルは、治療プロセスを大幅に簡素化するだけでなく、従来のCAR-Tに比べてコストを10分の1に削減することが期待されており、次世代の細胞免疫療法のブレークスルーとなるでしょう。
tLNP: 生体内CAR-Tのコアデリバリーシステム
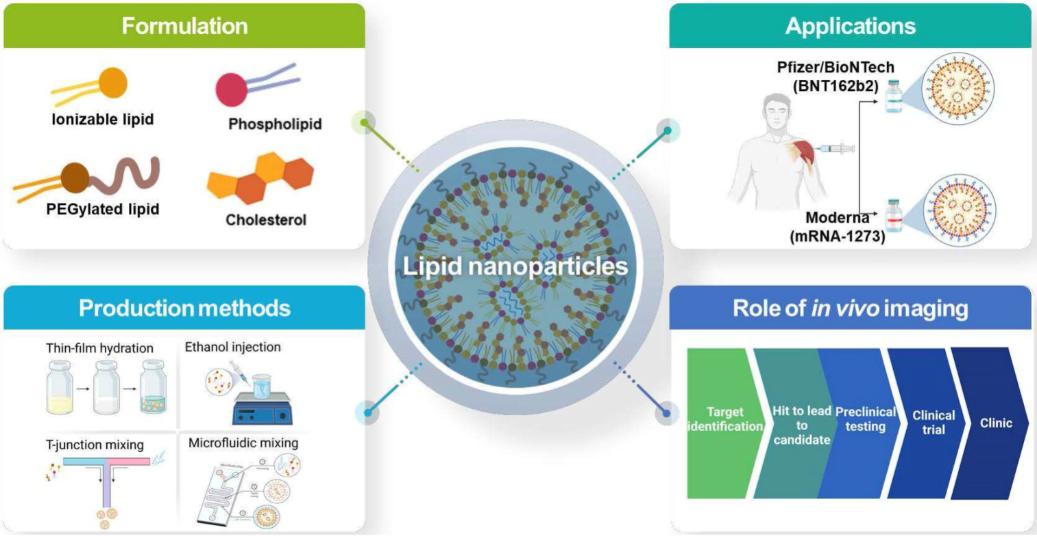
体内CAR-T療法の実施経路において、標的脂質ナノ粒子(tLNP)が業界の主流の技術ルートとなっています。tLNPは、特定の標的リガンド(抗体やペプチドなど)で表面を修飾することにより、従来のLNPに遺伝子カーゴを正確に認識してT細胞に送達する能力を与えます。
核となる技術は、標的抗体をLNP表面に精密に結合させ、「ナビゲーションミサイル」のような送達システムを構築することです。tLNPが体内に入ると、表面の標的リガンドがナノ粒子全体をT細胞に特異的に結合させ、エンドサイトーシスを介してCAR-mRNAを細胞内に送達することで、T細胞のin situリプログラミングを実現します。
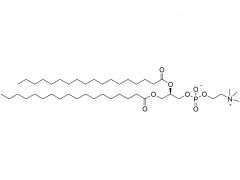
DSPE-PEG-MAL
: tLNP構築の鍵となる「化学の架け橋」
標的送達の推進:tLNP機能化の3つの強化
tLNP構築の複雑なシステムにおいて、DSPE-PEG-MALは動的な「実行者」としての役割を果たします。その構造設計は、調製プロセスにおける主要な機能と正確に一致しており、その役割は以下の3つの段階的かつ一貫したステップに分解できます。
· 埋め込みと固定:溶液から膜相までの正確な位置決め
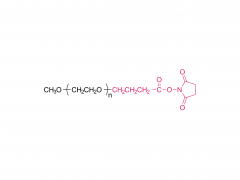
LNPの自己組織化プロセスにおいて、DSPE-PEG-MALは、DSPE疎水性末端の物理化学的特性を利用して、形成中の脂質二重膜上に自発的に「アンカー」することができます。このプロセスにより、分子全体が所定の配向と安定した状態でLNP構造の一部となり、その後の機能の基盤が築かれます。
· ストレッチと保護:インターフェース上に空間防御ラインを確立する
親水性PEG2000鎖は、LNP周囲の水性環境まで完全に伸び、柔軟で水和した「ブラシのような」保護層を形成します。この保護層はLNPの「第一防衛線」として機能し、複雑な生体内環境において、非特異的なタンパク質吸着を阻害し、粒子の凝集を抑制することで、LNPの物理化学的安定性と機能的完全性を維持し、体内での循環半減期を延長します。
· クリックとエンパワーメント:汎用キャリアから精密ミサイルへの化学変化
PEG鎖末端に位置するマレイミド(-MAL)基は、LNPに「インテリジェンス」を与える化学スイッチです。高度に選択的なバイオカップリング部位として、改変された抗体(例えば、ジスルフィド結合の還元やSATAなどの試薬を用いて導入)のチオール基と効率的に反応します。まさにこの不可逆的な共有結合段階を通して、通常のLNPSは「エンパワーメント」され、特定の細胞表面抗原を正確に認識できる標的TLNPSへと進化します。
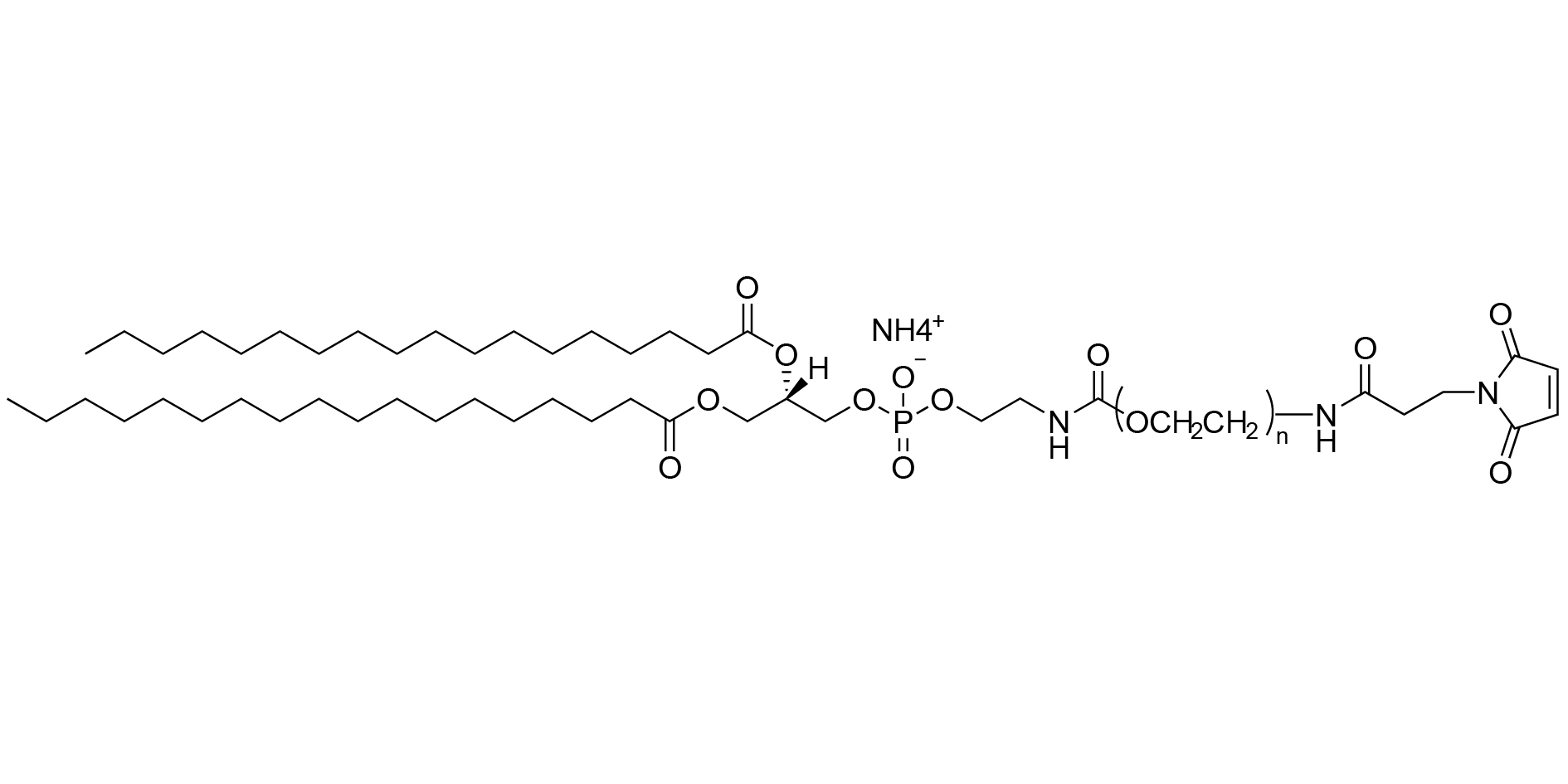
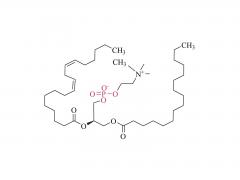
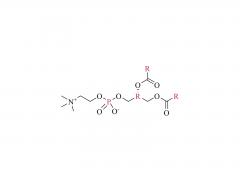
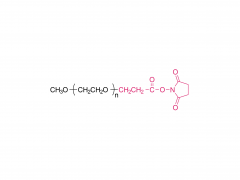
図1:DSPE-PEG-MAL(アンモニウム塩)の分子構造の模式図
LNP表面への抗体結合を実現するための技術的アプローチ
DSPE-PEG-MALを用いてLNP表面に抗体を結合させる方法は、現在tLNP構築において最も成熟し、広く適用されている戦略です。このプロセスは通常、以下の3つのコアプロセスで構成されます。
LNPの調製:DSPE-PEG-MALをマイクロ流体混合技術を用いて他の脂質成分(イオン化脂質、補助脂質、コレステロール、PEG脂質)と混合し、LNPを調製します。この際、DSPE-PEG-MALのマレイミド(-MAL)基がLNP表面に露出します。
2. 抗体の前処理:業界で標的 LNPS を構築するための一般的な方法(Capstan Therapeutics の特許 WO2024249954 に記載されている方法など)に従って、抗体をチオール(-SH)で修飾して、後続のカップリング反応を準備します。
3. カップリング反応:チオール(-SH)結合抗体を適切な緩衝液(PBS、pH 7.4など)中でLNPとインキュベートし、低温(例えば4℃)で(通常は数時間から一晩)反応させる。抗体のチオール基がLNP表面のマレイミド(-MAL)基と共有結合し、標的の機能化修飾を完了する。
DSPE-PEG-MALに基づくLNP送達システムは、レンチウイルスやアデノ随伴ウイルスなどのウイルスベクターと比較して、免疫原性が低く、挿入変異のリスクがなく、大規模生産が容易であるなどの利点があり、体内でのCAR-Tに最適なベクターとなっています。
SINOPEGのGMPグレードDSPE-PEG-MALの主な利点
GMPレベルの生産と即時供給
現在のin vivo CAR-Tの研究開発の波において、高品質で安定的に供給される主要添加剤は、研究開発の円滑な進展を確保するための礎となっています。厦門SINOPEGeは、完全なGMP品質管理システムを構築し、DSPE-PEG-MALのGMPレベルの大規模生産に成功し、十分なスポット在庫を構築しました。
CDEとDMFの二重申請がまもなく完了する
医薬品登録申請においてお客様をより良くサポートするために、弊社の DSPE-PEG-MAL 製品は積極的に宣伝されています。
· 中国CDE添加剤登録および申請:完了後、医薬品登録申請に直接関連付けることができます。
· 米国におけるFDA DMF申請:FDAに対して添加剤の品質とコンプライアンスを証明するための権威ある根拠を提供します。
SINOPEGは、原材料の調達から合成プロセス、精製管理に至るまで、包括的な品質管理システムを構築しています。in vivo CAR-Tのような最先端治療において、添加剤のわずかな違いが最終製剤の物理化学的特性、生体内挙動、さらには治療効果に甚大な影響を与える可能性があることを私たちは十分に認識しています。私たちは、純度、マレイミド(-MAL)基の活性、PEG鎖の完全性を厳密に管理するだけでなく、長年にわたるPEGおよび活性誘導体の工業生産に関する深い理解と、複数のINDプロジェクト申請の経験に基づき、一連の具体的な特性評価試験を開発しました。これらの詳細な物理化学的特性評価によって得られる差別化された利点は、お客様のプロジェクト申請に対し、より包括的なデータサポートを提供することができます。二重申請が完了すると、お客様は当社の申請番号を直接使用できるため、IND(新薬臨床試験申請)またはNDA(新薬販売承認申請)の申請プロセスが大幅に簡素化され、時間と人件費が節約され、プロジェクト申請の成功率が大幅に向上します。
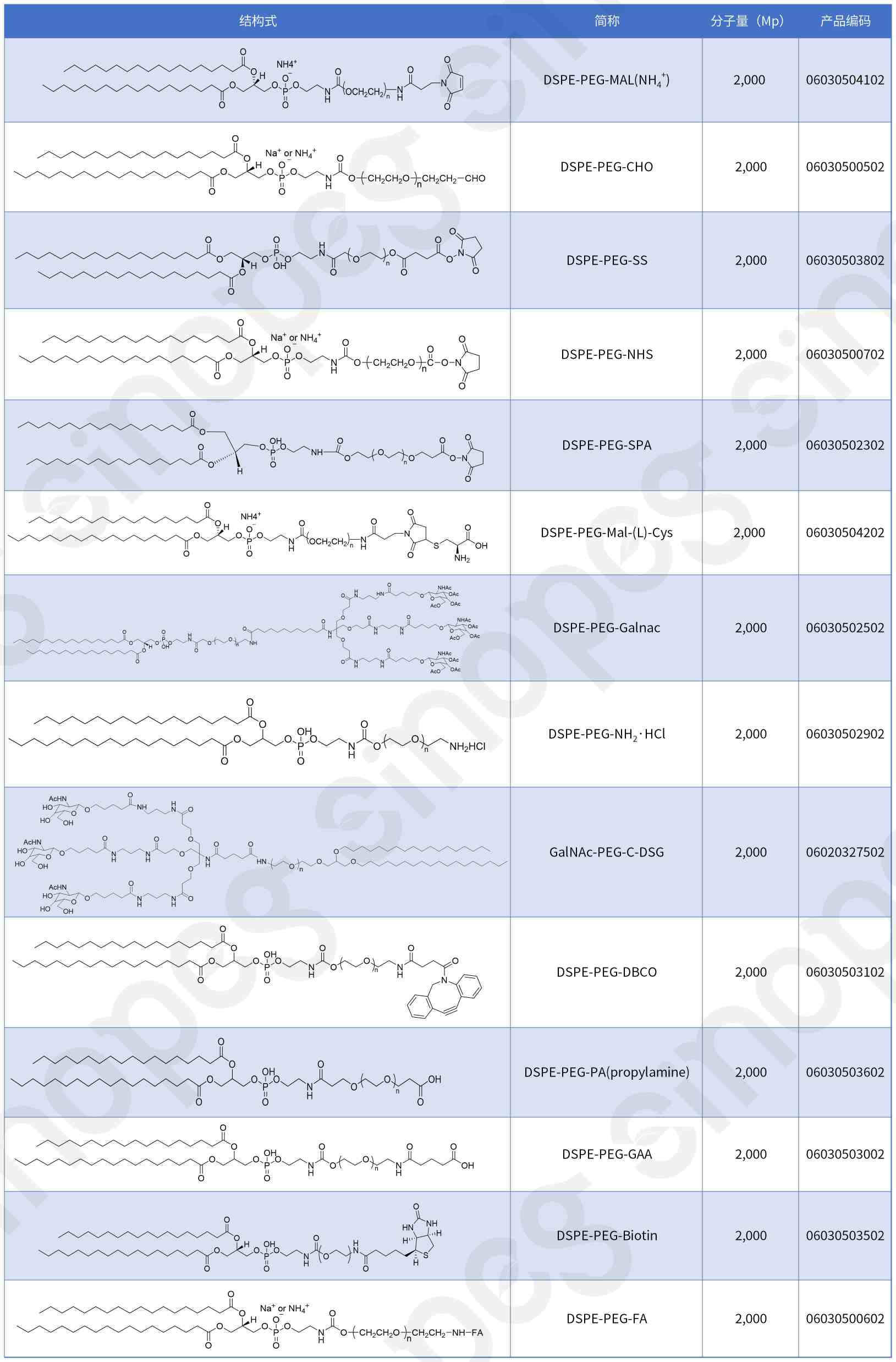
DSPE-PEGシリーズ製品の拡充
DSPE-PEG-MALに加えて、当社はさまざまなR&Dニーズを満たすために、さまざまな機能化リン脂質PEG誘導体のカスタマイズされた開発サービスも提供できます。
参照:
1. Capstan Therapeutics. Capstan Therapeutics、自己免疫疾患治療のためのリードとなるin vivo CAR-T療法CPTX2309の第1相試験の開始を発表。Business Wire. 2025年6月11日
2. AbbVie. AbbVie、Capstan Therapeuticsを買収、免疫学における患者ケアの変革への取り組みをさらに強化。AbbVie News Center. 2025年6月30日
3. 米国臨床腫瘍学会. CAR-T細胞療法:費用と検討事項. ASCO年次総会議事録. 2024.
4. Smith J, et al. 「生体内CAR-T細胞は製造の複雑さを軽減しながら強力な抗腫瘍活性を示す」Nature Biotechnology. 2023;41(5):678-685.
5. Wang D, et al. 生体内T細胞工学のための標的脂質ナノ粒子. Science Advances. 2024;10(12):eadl2165.
6. Chen X, et al. 抗体結合LNPを用いたT細胞への正確な生体内遺伝子送達 Cell Reports Medicine. 2024;5(3):101489.
7. Zhang Y, et al. DSPE-PEG脂質の分子構造と膜アンカー特性. Biochimica et Biophysica Acta. 2023;1865(4):184321.
8. Gabizon A, et al. ポリエチレングリコールコーティングリポソームに封入されたドキソルビシンの循環時間の延長と悪性滲出液への蓄積の促進. Cancer Research. 1994;54:987-992.
9. Hermanson GT. バイオコンジュゲート技術. 第3版. アカデミックプレス; 2021年.
10. Wei X, et al. T細胞工学のための標的脂質ナノ粒子のマイクロ流体合成. Lab on a Chip. 2024;24(8):2156-2168.
11. Kato T, et al. Traut試薬を用いた効率的な抗体結合によるLNP機能化. Bioconjugate Chemistry. 2023;34(7):1256-1265.
12. Li M, et al. 標的送達のためのLNP表面への抗体の共有結合.Journal of Controlled Release. 2024;368:456-467.
13. Xu Q, et al. 生体内CAR-T細胞工学のための非ウイルス性デリバリーシステム. Nature Reviews Materials. 2024;9(3):189-205.