I. 概要
H2N-PEG7-OH
特定の末端官能基を有するポリエチレングリコール誘導体です。その名称は化学構造をそのまま表しています。
H2N- : 片方の端のアミノ基
PEG7: 7つのエチレングリコール繰り返し単位からなるポリエチレングリコール鎖
-OH: 反対側の端にあるヒドロキシル基
したがって、これは異種二官能性架橋剤であり、2つの異なる末端を有し、それぞれが別々に化学反応を起こすことができます。そのため、異なる分子(例えば、カルボキシル基を持つ分子とアミノ基を持つ分子)を結合するための理想的な「ブリッジ」または「スペーサーアーム」となります。
II. 化学構造式
その線形化学構造は次のように表すことができます。
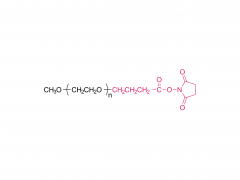
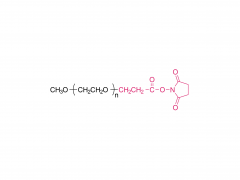
H₂N-CH₂CH₂-O-(CH₂CH₂-O)₆-CH₂CH₂-OH
あるいは、より簡潔に表現すると次のようになります。
NH₂-PEG₇-OH
ここで、PEG₇は7つの-ch ₂CH₂O-繰り返し単位を表します。
分子量:約365.4 g/mol
III. 主な特徴と利点
異種の二機能性
アミノ基は通常、生理学的条件下では正に帯電しており、活性化エステル、カルボキシル、カルボニルなどの基と反応してアミド結合またはその他の安定した結合を形成できます。
ヒドロキシル基は比較的不活性ですが、特定の条件下では活性化され、カルボキシル基とエステル結合を形成したり、他のヒドロキシル基と反応したりします。
優れた溶解性
PEG 鎖は親水性が強いため、H2N-PEG7-OH は水、緩衝液、多くの有機溶媒に対する溶解性が優れており、生化学反応への応用が非常に容易になります。
立体障害の遮蔽と半減期の延長:
薬物、ペプチド、またはタンパク質が PEG 鎖に結合している場合、PEG 鎖は分子の周囲に「水和層」を形成し、プロテアーゼなどの大きな分子からの攻撃を効果的に防御し、安定性が向上します。
改変された分子はサイズが大きくなり、腎臓の濾過が減少し、それによって体内の循環半減期が延長されます。
免疫原性を低減する
ペグ化により、生体分子(治療用抗体や酵素など)の表面にある抗原決定基をマスクできるため、免疫原性が低下し、抗体産生のリスクが軽減されます。
フレキシブルスペーサーアーム
7ユニットのPEG鎖は、十分に長く柔軟な空間距離を提供します。これにより、結合した2つの分子がそれぞれの活性を維持することができ、特に一方の分子が結合ポケットの奥深くに位置する標的と相互作用する必要がある場合に有効です。
IV. 主な応用分野
H2N-PEG7-OH は、主に以下のようなバイオコンジュゲート化学および材料科学の分野で広く使用されています。
ペグ化修飾
タンパク質/ペプチドの peG 化: タンパク質のアミノ末端をカルボキシル基と反応させるか、タンパク質のアミノ基をその活性化カルボキシル基と反応させることにより、タンパク質は peG 化によって修飾され、薬物動態特性が改善されます。
低分子医薬品のペグ化:低分子医薬品を PEG 鎖に結合して水溶性と生物学的利用能を向上させます。
生物学的接合と架橋
架橋剤として、カルボキシル基を持つ分子とアミノ基を持つ別の分子を結合させます。例えば、ビオチンと抗体を結合させたり、蛍光色素とオリゴヌクレオチドを結合させたりします。
材料表面の機能化
活性基を有する材料(シリカ、金ナノ粒子、ポリマーミクロスフェアなど)の表面に、ヒドロキシル末端を介して固定され、表面に遊離アミノ基を付与します。これらのアミノ基は、標的分子、蛍光プローブ、その他の機能性分子とさらに結合し、バイオセンサーや薬物送達キャリアなどを作製することができます。
デンドリマーおよびポリマーの合成:
構成要素として、精密な構造を持つより複雑なポリマー材料を合成するために使用されます。
V. 使用上の注意
保管方法:密閉し、乾燥した涼しい場所(通常は-20℃)に保管してください。吸湿性があるため、空気に触れると水分を吸収し、アミノ基の炭酸化などの副反応を引き起こす可能性があります。
反応性: アミノ基は空気中で酸化されやすいため、通常、反応は不活性ガスの保護下で実行する必要があります。
官能基の保護: 多段階合成において、アミノ基の活性を保持する必要がある場合、アミノ基を保護するために保護基を使用し、もう一方の末端での反応が完了した後に保護を除去する必要がある場合があります。
VI. 要約
H2N-PEG7-OHは、非常に重要かつ広く使用されている異種二官能性PEG架橋剤です。片端にアミノ基、もう一端にヒドロキシル基を有する独自の構造、優れた水溶性、生体適合性、そして柔軟なスペーサーアームとして機能する能力により、医薬品開発、バイオコンジュゲーション化学、ナノテクノロジー、材料科学などの分野で不可欠な役割を果たしています。複雑な生物学的コンジュゲートや機能化材料を構築するための強力なツールとして、研究者にとって強力なツールとなっています。